Retooling Protein Characterization
Dr. Lisa Heiden, Genetic Engineering & Biotechnology News - February 15, 2016
If The Biotherapeutics Workshop Is to Progress beyond Rough-Hewn Products, It Will Need a Range of Finely Calibrated Analytical Platforms
Protein characterization approaches go hand in hand with analytical instrumentation platforms. This point was expressed in various ways by the presenters at Peptalk: The Protein Science Week, a Cambridge HealthTech Institute event recently held in San Diego. The event’s speakers and presenters also emphasized how protein characterization could advance specific research areas.
“Biotherapeutics, ranging from insulin to antibodies to viruses to siRNA, are interesting molecular tools to address a wide range of diseases,” said Wafa Hassouneh, Ph.D., applications scientist, Wyatt Technology. “In the quest to develop effective and robust biotherapies, novel molecules have to be characterized to determine their properties and behavior.”
Our increasing knowledge of cell signaling pathways and mechanisms is opening up a plethora of opportunities for novel target-based biotherapeutic drugs. “I think everyone agrees harnessing the patient’s immune system is powerful,” declared Gunnar F. Kaufmann, Ph.D., senior vice president, Sorrento Therapeutics. “We are now getting to the point where we can make therapeutics targeting immune pathways.”
The complexity of targets is increasing with the shift toward more complicated therapeutic intervention, such as bispecific antibodies (BsAbs). Emerging technologies offer significant functional advantages, such as synergistic mechanism of action and increased selectivity. They are also “putting an increasing emphasis on more detailed biophysical characterization studies,” suggested Paul Belcher, Ph.D., functional leader, Biacore, GE Healthcare.
Stabilizing Membrane Proteins
Integral membrane proteins (IMPs), central players in cell signaling pathways, are therapeutic targets for a significant percentage of approved protein drugs. Yet targeted IMPs are often not fully characterized due in part to challenges in preserving native, functional conformations. High concentrations of detergents required for maintaining IMP solubility in aqueous solutions typically interfere with protein characterization methods.
Nanodisc technology is a synthetic model membrane system increasingly being employed for overcoming associated IMP challenges. Han Xu, Ph.D., principal scientist, Amgen, discussed nanodisc as a reliable and enabling method for solubilizing and stabilizing IMPs into detergent-free lipid-bilayer environments.
The IMP is stabilized within the nanodisc structure “by a pair of membrane scaffold proteins and the lipid bilayer, which mimics the endogenous cellular membrane without detergent interference,” explained Dr. Xu. “Thus, IMPs are likely to be presented in their native form or in a form very close to their native form in nanodisc.” “This approach,” he continued, “enables a wide range of detection methods such as SPR (surface plasmon resonance), BSI (back scattering interferometry), and AUC (analytical ultracentrifugation) to be employed for detailed biophysical and biochemical characterization of membranes.”
Dr. Xu noted that he had determined binding properties of peptide and small molecule inhibitors of Kv.1.3. He reported that he had used a chimeric ion channel, Kcs-Kv.1.3 nanodisc, and direct binding technologies. His results, he asserted, would have been impossible using micelles or liposomes. Kv.1.3, an important therapeutic target, is a potassium voltage-gated ion channel with key roles in regulating activation of immune response signaling pathways. Although Kv.1.3 function remains to be fully elucidated, dysregulation is implicated in chronic inflammatory syndromes. There is considerable interest in developing more specific Kv1.3 inhibitors for attenuating immune responses.
Retooling Monoclonals into Bispecifics
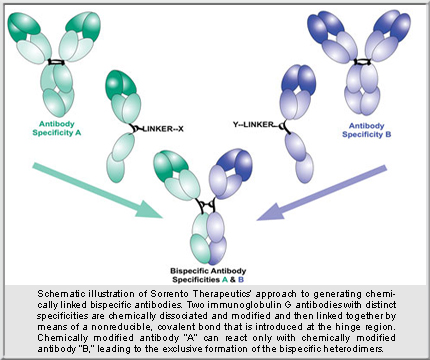
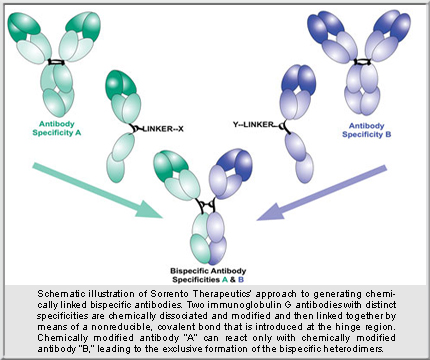
Most BsAb technologies require genetic/protein engineering to fuse antibody (Ab) specificities together. Sorrento’s approach, however, involves “an off-the-shelf approach,” said Dr. Kaufmann. “We make IgG-like BsAbs utilizing proteins we already have.” Any two homodimeric Abs can be cut in half and joined together. Many companies already have plenty of antibodies, so there is no need to reinvent the wheel, said Dr. Kaufmann. “Just tweak already developed antibodies,” he suggested.
Chemically modified Ab halves can’t react with each other but can react with another specificity’s half, forming BsAbs having properties the two parental Abs had as a mix, but not individually. “BsAbs binding to both targets at the same time have much stronger binding than either parental Ab,” Dr. Kaufman points out. Yanwen Fu, Ph.D., associate director of antibody technologies and chemical biology at Sorrento, described how easily research tools could be developed: “You can quickly combine any two specificities together and see what they do.” Comprehensive protein characterization ensures the BsAb is suitable “not only for discovery, but also for future process development,” Dr. Fu emphasized.
Cancer cells evolve mechanisms to undermine immune system networks and trigger shutdowns, like PD-L1 upregulation, which downregulates cytotoxic T cell activity and dampens immune responses. The c-Met /PD-L1 BsAb is designed to block aberrant c-Met phosphorylation/activation signaling while activating immune responses. “It is critical to show that BsAbs keep the integrity and natural properties of parental antibodies,” Dr. Fu insisted. Flow cytometry of surface-bound c-Met/PD-L1 bispecific-IgG on breast cancer cells verified strong binding affinity, and ELISAs showed that this BsAB suppressed c-Met phosphorylation induced by hepatocyte growth factor, validating c-Met blocking activity. PD-L1 blocking activity was verified in immunomodulatory assays with T and antigen-presenting cells wherein the BsAb increased IL-2 release, a T cell activity marker.
In vivo animal models will aim to show that the BsAb induces synergistic anticancer activity by inhibiting c-Met signaling while inducing immune-mediated cell killing. “We are always happy to partner with others who have Abs they want to turn into BsAbs,” informed Dr. Kaufmann.
Applying New Tools and Techniques
Applied scientific knowledge and analytical instrument platform capabilities are inextricably interwoven. Philip Chapman, product manager at Bio-Rad Laboratories, polled customers, asking, “What was it that swung you to buy your chromatography system from us?”
“The number one answer we got was the software,” Mr. Chapman said. The software, he asserted, is intuitive, easy to use, contains method/application templates, and is integral to the modular design of the company’s next-generation chromatography (NGC™) system.
Dr. Hassouneh discussed Wyatt Technology’s suite of analytical tools, which are also detailed in the company’s webinars. “These tools help answer various questions,” she maintained. “What biotherapeutic is synthesized? What post-translational modifications, impurities, or degradants are present? How does the biotherapeutic behave in different conditions? How robust it is during scale up? What interactions does it have with targets?”
Weighing in on techniques, Dr. Belcher pointed out that “over 60% of approved antibody therapeutics have used Biacore SPR either in research and development, R&D filing (investigational new drugs), or BLA (biological licensing agreement).” After stating that Biacore’s DiPIA [Developments in Protein Interaction Analysis] online community and biennial conferences are available as venues for exchanging scientific expertise, he highlighted one particular upcoming event: “SPR in the drug discovery workflow will be covered at DiPIA 2016, an event for SPR practitioners that will be held in June in Berlin.”
“No one biophysical technique can answer all the questions for a given CQA (critical quality attribute),” Dr. Belcher added. “Protein characterization requires a whole suite of techniques.”
Seeing the (Scattered) Light
DLS (dynamic light scattering) assesses size distribution, and MALS (multi-angle light scattering) measures molar mass. Both are utilized throughout drug discovery, beginning with quality assessments of proteins to identify the best candidate molecules.
“Binding studies are no better than the quality of the solutions and molecules used in the studies,” Dr. Hassouneh warned. She proposed using Wyatt’s DynaPro® DLS Plate Reader, which is, she said, capable of checking for aggregates in seconds: “You can run all your solutions through it to identify aggregated solutions before they are inadvertently loaded onto delicate instruments.”
“Promising biotherapeutic candidates are tested for stability and robustness as they are pushed further down the pipeline,” noted Dr. Hassouneh, who added that thousands of conditions could be screened per day with DynaPro to determine aggregation and stability of the biotherapeutic.
Wyatt’s MALS detectors (DAWN®, miniDAWN®, and microDAWN™) are used in conjunction with chromatography to determine the absolute molar mass, size, conformation and/or conjugation of macromolecules such as proteins without reference to molecular standards. MALS is combined with their Calypso® composition gradient to characterize biomolecular interactions. “Calypso is a great label-free, immobilization-free tool to characterize simple or complex biomolecular interactions,” Dr. Hassouneh exclaimed.
The ASTRA® software package leverages instruments’ capabilities, providing powerful analysis tools including the protein conjugate analysis tool, which enables determination of molecular weight distributions of PEGylated proteins, antibody glycosylation status, or drug-to-antibody ratio.
Real-Time Reporting
Biacore uses the biophysical phenomenon of SPR to detect and characterize biomolecular interactions in a real-time, label-free, and contact-free environment. Real-time allows one to see the entire event as it occurs, not just a snapshot or endpoint, as in ELISA. Real-time measures the kinetics of interactions such as how quickly drugs recognize their protein targets or how long they occupy receptors. “Kinetics has therapeutic consequences affecting multiple aspects of drug function such as dosing and pharmacokinetics,” says Dr. Belcher. “Kinetics allows the linking of structure to function, allowing a greater understanding of biological mechanisms.”
“Contact free” means the detection system is, on principle, outside of where protein interactions take place. This enables one to work with opaque samples or complex mixtures such as crude antibody hybridoma supernatant or even blood, making it highly suitable to process development workflows. The FDA requires biosimilars to be highly similar to the innovator product, with no clinically meaningful differences in terms of safety, purity, and potency. “Contact free” enables crude protein products to be analyzed early on against the innovator molecule, allowing the identification of problems much earlier in the production process.
Biacore added a comparability function to its platform in 2015, allowing direct statistical comparisons of one sample to another. The comparability software enables drug companies to assess how similar their fledging products are to the innovator partner they are hoping to compete against. “It overcomes the challenge of characterizing kinetic interactions that are not a simple 1:1,” concluded Dr. Belcher. “Our comparison tool enables that because it provides a direct statistical comparison. It doesn’t require a mathematical model, meaning it’s always applicable.”
Streamlining Separation
Moving from a single column—bind, elute, and analyze your fraction—to more multicolumn applications or automated batch purifications is part of next-generation chromatography. Chromatography, based on size separation, is an essential protein purification tool. For downstream characterization processes, it’s critical “to ensure that you’re looking at what you intended to look at, and that you haven’t co-purified something affecting protein integrity,” said Jeff Habel, Ph.D., applications manager, Bio-Rad Laboratories.
Traditional chromatography was labor intensive. Skilled, experienced people were essential because it was so difficult to learn. Previously, even a simple His-tag purification took days. To accelerate such separations, Bio-Rad developed a next-generation chromatography system that it calls NGC.
NGC is designed to automate processes and effectively reduce days to hours. The idea is to let users load samples, press a button, and walk away to do other work while the entire purification process happens by itself. According to Bio-Rad, NGC automates processes for empirically determining optimal purification conditions. For example, NGC can prepare a range of buffers. “Just specify the salts and pH you want, and the automation scouts through a range of different conditions during the run,” explained Mr. Chapman. Then, he continued, the user may overlay the different chromatograms to identify the separation’s optimal conditions. The modularity of the system enables columns and pumps to be switched out as the user goes from pilot studies, which involve purifying small amounts of protein, to scale up efforts, which require larger columns.
The NGC “open the box and start to use it” approach is meant to make chromatography accessible to more people. “Smaller research groups or for-profit labs can now do their own purification work as part of their work flow,” asserts Mr. Chapman. NGC is designed to be customizable. For example, instrument modules can be matched to customer requirements. As requirements expand, “just drop in new modules,” Mr. Chapman advised. “The software recognizes them, and the functionality is immediately there.”
As published in GEN Engineering News: Retooling Protein Characterization
